- PRODUCT DETAILS
- RELATED PRODUCTS
PRODUCT DETAILS
1. Product Introduction
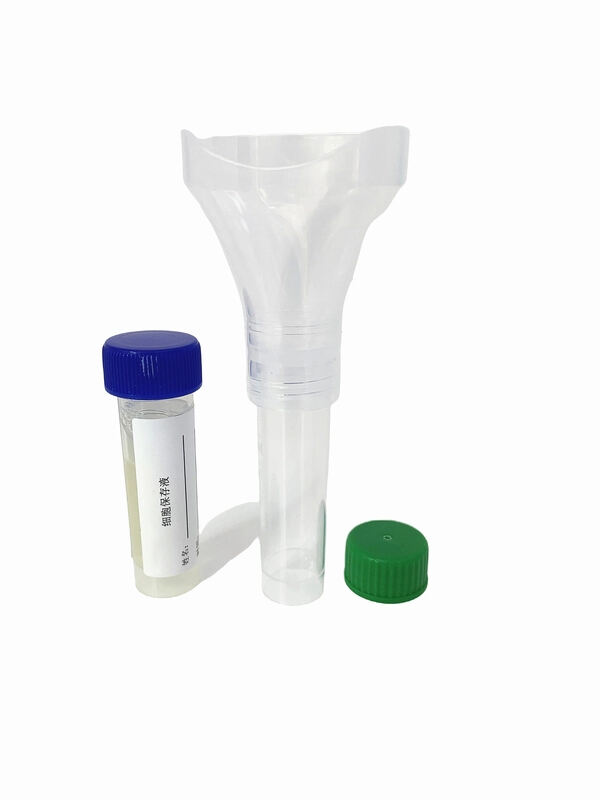
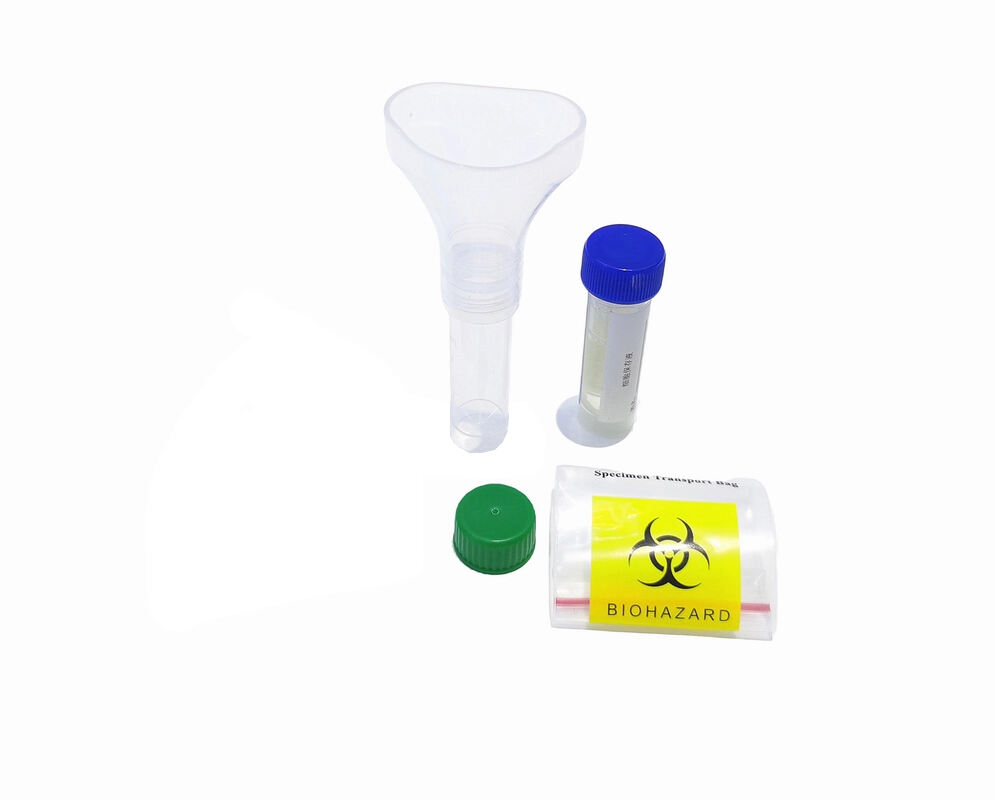
- Product Overview: The Saliva Collection Kits developed by Xiamen Zhizi Industry & Trade Co., Ltd. are professional medical consumables designed for non-invasive saliva sample collection, preservation, and transportation. Widely used in nucleic acid testing (e.g., COVID-19, influenza), genetic analysis, and clinical research, these Saliva Collection Kits replace traditional invasive sampling methods (e.g., nasopharyngeal swabs), significantly improving subject compliance. Each kit integrates a collection tube, pre-filled preservation solution, sterile swab, and instruction manual, providing a one-stop solution for safe and efficient saliva sampling.
- Product Performance: The Saliva Collection Kits excel in sample stability, usability, and compatibility. The pre-filled saliva preservation solution contains nuclease inhibitors and proteinase K, maintaining nucleic acid integrity for up to 14 days at 2-25℃ (detection rate ≥99%). The collection tube, made of medical-grade PET material, is highly transparent (light transmittance ≥92%) for easy sample observation. The kits support seamless connection with automated nucleic acid extractors (e.g., Thermo Fisher KingFisher) and comply with global medical standards, ensuring reliable performance in diverse testing scenarios.
2. Product Advantages
- Full-Process Traceability System for Data Integrity: Both the collection tube and transport tube of the Saliva Collection Kits are pre-reserved with QR code areas, supporting laser coding (each kit has a unique 16-digit code). The QR code is linked to key information (batch number, production date, sterilization record) in the backend system. When connected to Laboratory Information Management Systems (LIMS), it enables real-time recording of sampling time, operator, transport route, and testing results. This full-chain traceability not only avoids sample confusion but also meets the data audit requirements of medical institutions and regulatory authorities, ensuring accountability for every step of the sampling-detection process.
- Multi-Scenario Adaptive Packaging for Diverse Needs: The Saliva Collection Kits offer three tailored configurations to match different application scenarios. The Home Self-Test Version includes a detailed graphic manual (with step-by-step sampling instructions in 6 languages) and a disposable collection cup, enabling users to complete sampling independently without professional guidance. The Medical Institution Version adopts trace-free sterilization packaging (using ethylene oxide sterilization, residue <10μg/g) to avoid cross-contamination in high-throughput testing environments. The Epidemiological Survey Version is pre-equipped with a cryopreservation tube (tolerating -80℃ freezing) for long-term sample storage, suitable for large-scale population surveys requiring delayed testing.
- International Compliance Certifications for Global Application: The Saliva Collection Kits have obtained CE IVDR certification (compliant with EU in vitro diagnostic regulations) and FDA 21 CFR 820 quality system certification, meeting strict market access standards in Europe and North America. Their biosafety performance complies with ISO 13485, with no cytotoxicity, skin sensitization, or irritation (verified by third-party testing). Additionally, the kits support both global cold-chain (2-8℃) and room-temperature (2-30℃) logistics solutions—their packaging is designed with moisture-proof and shock-resistant layers, ensuring reagent stability during long-distance transportation (e.g., transoceanic shipping), facilitating global supply.
3. Product Production Process
- Material Preparation and Inspection: For manufacturing the Saliva Collection Kits, high-purity medical-grade PET (for collection tubes) and PP (for swabs) are selected. Each batch of materials undergoes 12 quality inspections (including chemical composition, biocompatibility) to ensure compliance with medical standards. The preservation solution is formulated in a 100,000-level clean room, with raw materials (nuclease inhibitors, proteinase K) sourced from certified suppliers. Only qualified materials are used to produce the Saliva Collection Kits, laying a solid foundation for product quality.
- Precision Molding and Assembly: The collection tubes of the Saliva Collection Kits are produced via precision injection molding (tolerance ≤0.1mm), ensuring uniform wall thickness and stable structure. The swabs are manufactured with flocked tips (500 fibers/mm²) to enhance sample absorption. In the assembly workshop (class 10,000 clean room), workers wearing sterile suits assemble the Saliva Collection Kits—placing the pre-filled preservation solution into collection tubes, pairing with swabs and manuals, and sealing into individual packages. Each step is monitored by HD cameras to avoid assembly errors in the Saliva Collection Kits.
- Sterilization, Coding, and Final Inspection: After assembly, the Saliva Collection Kits undergo ethylene oxide sterilization (sterility assurance level ≥10⁻⁶). Then, laser coding equipment prints unique QR codes on the collection tubes of the Saliva Collection Kits. The final inspection includes leak testing (1.5MPa water pressure), reagent activity detection, and packaging integrity checks. Only the Saliva Collection Kits that pass all inspections are labeled with batch numbers and expiration dates, ready for storage and shipment. This strict process ensures consistent quality of every Saliva Collection Kits.
4. Frequently Asked Questions
Q: How long can the Saliva Collection Kits be stored after opening?
A: Once the outer packaging of the Saliva Collection Kits is opened, it is recommended to use the kit within 24 hours. If not used immediately, store the opened Saliva Collection Kits in a cool, dry place (2-8℃) and avoid exposure to sunlight or moisture to prevent reagent deterioration.
Q: Is the Saliva Collection Kits suitable for children under 3 years old?
A: The Saliva Collection Kits are designed for people aged 3 and above. For children under 3 years old (who cannot spit independently), we recommend using the kit under medical guidance—collecting saliva with a sterile swab and transferring it to the collection tube of the Saliva Collection Kits to ensure sample quality.
Q: Do the Saliva Collection Kits require cold-chain transportation?
A: No, the Saliva Collection Kits support both cold-chain (2-8℃) and room-temperature (2-30℃) transportation. Their preservation solution and packaging are optimized for room-temperature stability, reducing logistics costs. However, if transportation exceeds 7 days, cold-chain transportation is recommended to maintain optimal reagent activity.
If you are interested in our Saliva Collection Kits and want to know more about product specifications (e.g., preservation solution volume, packaging quantity), wholesale prices, or customization services (e.g., multi-language manuals, brand printing), please leave your inquiry information (name, company name, contact number, specific needs) on our official website. Our professional team will reply to you within 24 hours, providing detailed technical documents and tailored procurement plans.