- PRODUCT DETAILS
- RELATED PRODUCTS
PRODUCT DETAILS
1. Product Introduction
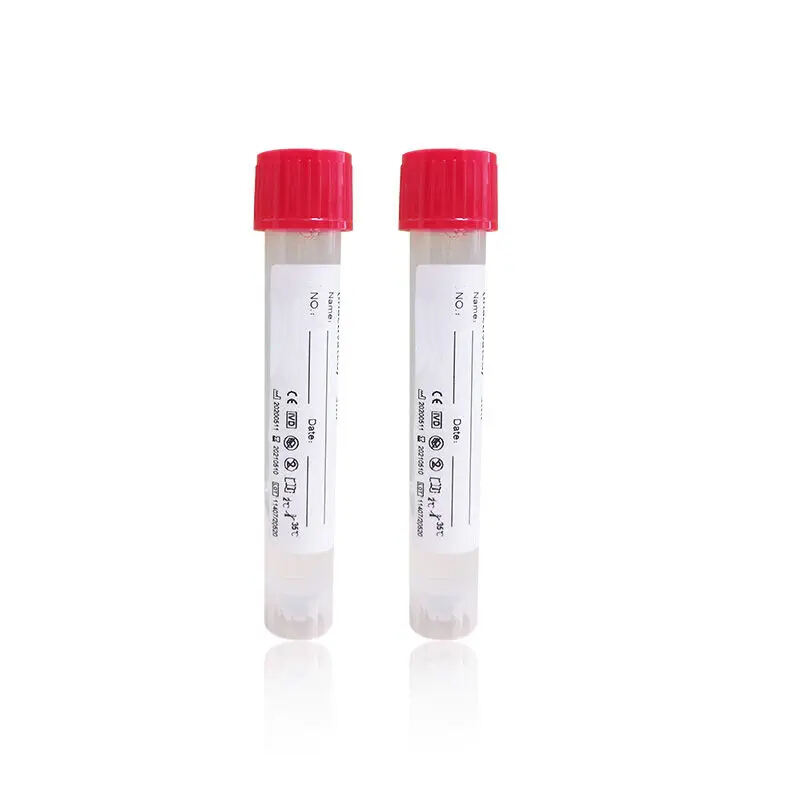
- Product Overview: The Nucleic Acid Sampling Tube developed by Xiamen Zhizi Industry & Trade Co., Ltd. is a professional medical device designed for nucleic acid sample collection, preservation, and transportation in clinical diagnosis, epidemiological surveys, and biological research. It integrates sample collection, reagent pre-filling, and contamination prevention functions, replacing traditional separate "swab + tube + reagent" combinations. Widely used in hospitals, testing laboratories, and disease control centers, this Nucleic Acid Sampling Tube ensures the integrity of nucleic acid samples while optimizing workflow efficiency, becoming a core consumable for accurate nucleic acid detection.
- Product Performance: The Nucleic Acid Sampling Tube excels in compatibility, safety, and traceability. With a standardized size of 13*100mm, it is fully compatible with mainstream nucleic acid extractors (e.g., Qiagen QIAcube, Thermo Fisher KingFisher). The pre-filled virus preservation solution maintains nucleic acid stability for 72 hours at 2-30℃, with a detection rate of over 98%. The tube body is made of medical-grade PET material, which is non-toxic, non-adsorptive, and resistant to 121℃ autoclaving. Equipped with a unique ID code and a barcode area, it supports full-process traceability, meeting IVDR/CFDA compliance requirements for medical devices.
2. Product Advantages
- Automation Compatibility Design for Seamless High-Throughput Testing: The Nucleic Acid Sampling Tube adopts a standardized size of 13*100mm, which is calibrated to match the adapter specifications of mainstream nucleic acid extractors on the market. This eliminates the need for replacing instrument accessories or adjusting parameters, enabling direct loading of the tube into the extractor for automated processing. The tube body features a dedicated barcode area that supports laser engraving of sample information (e.g., sample ID, collection time). When paired with automated scanning equipment, it realizes seamless connection between sample tracking and high-throughput testing. For laboratories handling over 1000 samples daily, this design reduces manual information entry time by 40%, minimizes human error (error rate ≤0.5%), and ensures efficient operation of the entire testing workflow.
- Humanized Operation Design for Enhanced Safety & Convenience: The Nucleic Acid Sampling Tube is optimized for user-friendly operation. Its screw cap is designed with anti-slip corrugations, allowing medical staff to easily twist it open or close with a single gloved hand—critical for scenarios where both hands are occupied or when wearing thick protective gloves. The inner cap is pre-installed with an anti-splash diaphragm made of food-grade silicone. When opening the cap, the diaphragm automatically scrapes off residual liquid on the swab or tube mouth, preventing aerosol formation and reducing the risk of cross-contamination by 80% compared to traditional tubes. Additionally, the tube mouth has a 3mm chamfered edge, which avoids hand scratches during sample addition and ensures smooth swab insertion/removal.
- Full-Process Traceability System for Compliance Assurance: Each Nucleic Acid Sampling Tube is assigned a unique ID code (laser-engraved on the tube body), which is linked to the product’s batch number, production date, and sterilization information in the backend system. When used with a Laboratory Information Management System (LIMS), the ID code can record key data throughout the sample lifecycle, including sampling personnel, collection time, transportation route, and testing results. This full-process traceability not only meets the strict compliance requirements of IVDR (In Vitro Diagnostic Regulation) and CFDA (China Food and Drug Administration) but also enables quick recall of problematic products in case of quality issues. For medical institutions and testing laboratories, this system provides a reliable audit trail, enhancing trust in the accuracy and safety of the testing process.
3. Product Operation Guide
- Step 1: Unpacking and Preparation: Take out the Nucleic Acid Sampling Tube from the sterile aluminum-plastic packaging, check if the Nucleic Acid Sampling Tube is intact (no cracks, no reagent leakage), and confirm the unique ID code matches the batch record. Put on disposable gloves and a face mask, then place the Nucleic Acid Sampling Tube on a clean, flat operating table to avoid contamination.
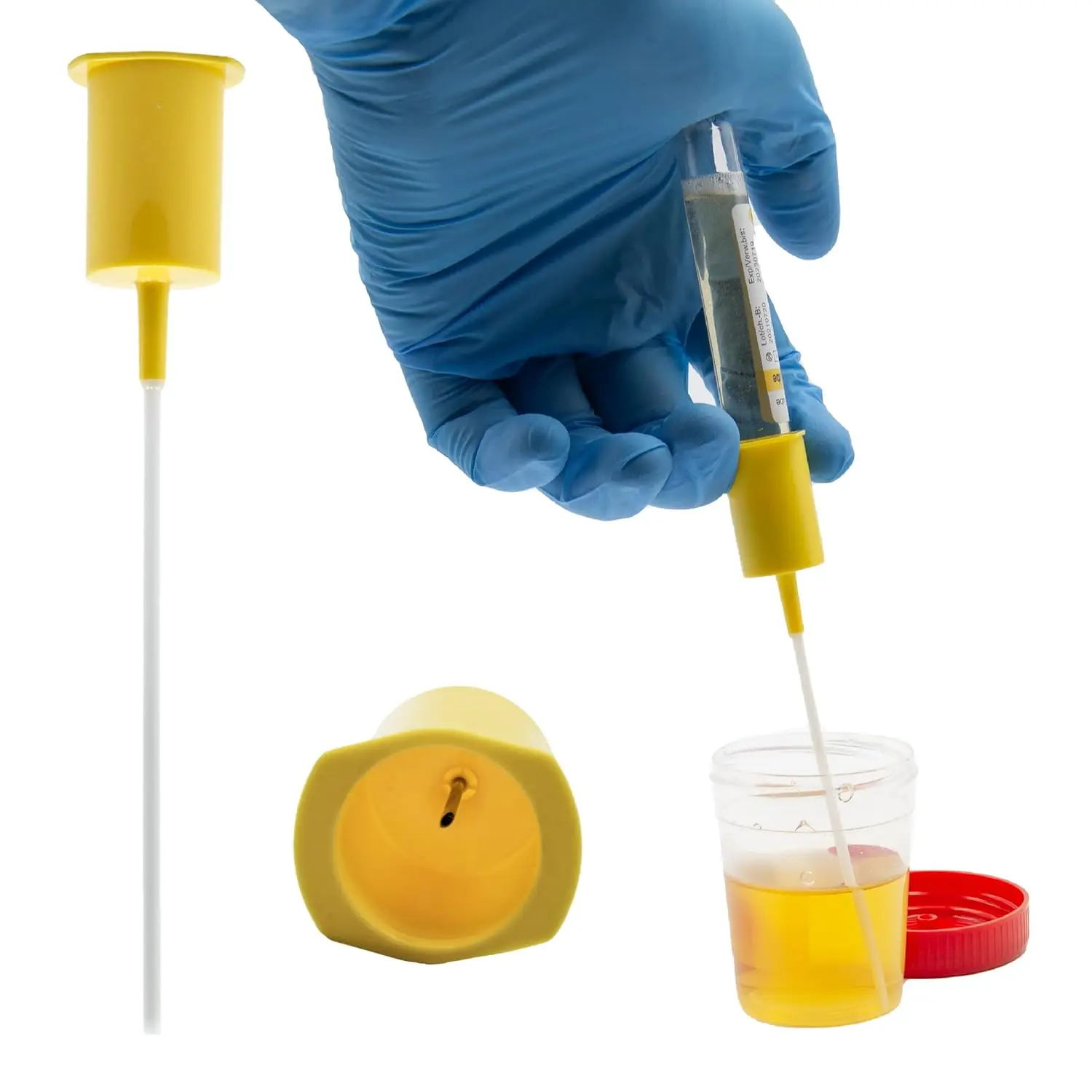
- Step 2: Sample Collection: Hold the Nucleic Acid Sampling Tube in one hand, twist open the screw cap (using anti-slip corrugations for easy operation), and take out the built-in sterile swab. Gently insert the swab into the patient’s nasopharynx or oropharynx to collect the sample, then return the swab to the Nucleic Acid Sampling Tube, breaking the swab rod at the marked line to ensure it is fully immersed in the preservation solution.
- Step 3: Sealing and Labeling: Screw the cap of the Nucleic Acid Sampling Tube tightly until a "click" sound is heard (indicating a secure seal), then wipe the outer surface of the Nucleic Acid Sampling Tube with 75% alcohol for disinfection. Use a laser scanner to read the barcode on the Nucleic Acid Sampling Tube, linking it to the patient’s information in the LIMS system, or manually mark the patient’s name and collection time on the tube’s information area.
- Step 4: Transportation and Detection: Place the sealed Nucleic Acid Sampling Tube into a biohazard transport box with ice packs (maintaining 2-8℃ if transportation exceeds 4 hours). Upon arrival at the laboratory, take out the Nucleic Acid Sampling Tube, confirm the ID code again, and load it into the nucleic acid extractor (compatible with 13*100mm adapters) for automated extraction and subsequent PCR detection. After use, discard the Nucleic Acid Sampling Tube into a medical waste bin for centralized disposal (do not reuse the Nucleic Acid Sampling Tube).
4. Frequently Asked Questions
Q: Can the Nucleic Acid Sampling Tube be used for both nasopharyngeal and oropharyngeal sampling?
A: Yes, the Nucleic Acid Sampling Tube is compatible with both nasopharyngeal and oropharyngeal sampling. The built-in swab is designed with a soft, flocked tip that is suitable for both anatomical sites, and the preservation solution can effectively stabilize nucleic acids from either type of sample without affecting detection results.
Q: What is the shelf life of the Nucleic Acid Sampling Tube, and how should it be stored?
A: The unopened Nucleic Acid Sampling Tube has a shelf life of 18 months when stored at 2-30℃ in a cool, dry environment away from direct sunlight and high humidity. Do not freeze the Nucleic Acid Sampling Tube, as freezing may damage the preservation solution and cause leakage.
Q: Is the Nucleic Acid Sampling Tube compatible with third-party LIMS systems, or only with specific software?
A: The Nucleic Acid Sampling Tube’s unique ID code and barcode follow international standard formats (e.g., Code 128), making it compatible with most third-party LIMS systems (e.g., LabWare, Waters Empower). Our technical team can provide a data interface manual to assist with system integration, ensuring smooth data transmission and traceability.
If you are interested in our Nucleic Acid Sampling Tube and want to know more about product specifications (e.g., preservation solution volume, packaging quantity), wholesale prices, or customization services (e.g., custom barcode formats, brand printing), please leave your inquiry information (name, company name, contact number, and specific needs) on our official website. Our professional sales team will reply to you within 24 hours, provide detailed product technical documents, and tailor a cost-effective procurement plan for your institution.